SAN DIEGO– The investigative oral Bruton’s tyrosine kinase (BTK) inhibitor remibrutinib reveals guarantee in clients with moderate to extreme hidradenitis suppurativa (HS), arises from a randomized 16-week stage 2 trial revealed.
“Research reveals that the TNF-alpha and IL-17 signaling paths have essential functions in HS,” lead detective Alexa B. Kimball, MD, MPH, from the Clinical Laboratory for Epidemiology and Applied Research in Skin at Beth Israel Deaconess Medical Center, Boston, stated at the yearly conference of the American Academy of Dermatology. “However, a number of extra paths are believed to add to illness pathogenesis.”
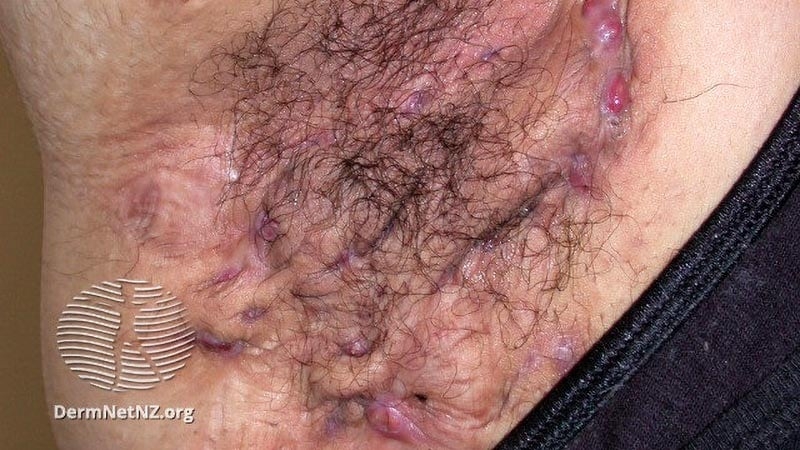
The existence of B cells and plasma cells has actually been reported in HS sores, she continued, consisting of early sores, with BTK activation as a main signal transduction path. For the existing research study, Kimball and associates examined the security and effectiveness of remibrutinib (LOU064), an oral, extremely selective BTK inhibitor, in 77 grownups with moderate to extreme HS for a minimum of 12 months in 2 or more physiological locations with 15 or less tunnels below the skin.
There were somewhat more females than males and more than 90% of research study individuals were White. The unique drug, which is being established by Novartis, is likewise under examination in other immune-mediated inflammatory illness, consisting of persistent spontaneous urticaria and numerous sclerosis.
Of the 77 clients, 33 were appointed to get 100 mg remibrutinib two times each day, 33 got a 25 mg twice-daily dosage, and 11 clients got placebo two times daily. The main endpoint was the percentage of clients who attained a streamlined Hidradenitis Suppurativa Clinical Response (HiSCR) at week 16 compared to pooled placebo. A streamlined HiSCR reaction was specified as a minimum of a 50% decrease in overall inflammatory abscess and blemish (AN) count, without any boost in draining pipes tunnels relative to standard.
Kimball, who is likewise a teacher of dermatology at Harvard University, reported that 80.2% of clients general finished treatment: 87.9% and 78.8% in the remibrutinib 25 mg and 100 mg arms, respectively, and 76% in the pooled placebo arm. The primary factor for treatment discontinuation was patient choice (60.9%). Almost 3 quarters of clients in the remibrutinib 25 mg twice-daily arm (72.7%) accomplished the streamlined HiSCR endpoint, compared to 48.5% of those in the remibrutinib 100 mg twice-daily arm, and 34.7% of those in the placebo arm.
In other exploratory findings, HiSCR, HiSCR 75, and HiSCR 90 rates were greater at week 16 amongst clients in both remibrutinib treatment arms compared to placebo, and the research study drug likewise was related to a higher impact on decrease of the AN count and draining pipes tunnels. Particularly, the approximated mean portion decrease in AN count was 68% in the 25 mg twice-daily arm, compared to decreases of 57% in the 100 mg twice-daily arm and 49.7% in the placebo arm, respectively. The approximated mean decreases in draining pipes tunnels were 55.6%, 43.6%, and 10.2%, respectively, in the 3 arms.
The scientists likewise observed a higher reaction on the Patient’s Global Assessment of Skin Pain Numeric Rating Scale 30 (NRS30) in clients treated with remibrutinib compared to those on placebo at week 16 (57.1% in the 100 mg twice-daily arm,